
By Mark Miller, CEO, Coating Tech Slot Dies LLC
Introduction

Typically, when you coat a fluid out onto a substrate, the fluid flows to the limits of the coating head and provides a solid layer of fluid over the substrate. Sometimes, however, the fluid resists this reformation and coating defects occur. Pinholes, ribs and beading-up of the fluid are common when the fluid resists flow. These coating defects are an example of the fluid liking itself more than it likes the substrate. Because of the fluid’s fondness for itself (chemically speaking, of course), the fluid contracts, or beads up. We have all seen this – water droplets form on a window and don’t want to wet-out, so they form individual beads (see Figure 1). In the coating world, though, we need the fluid to like the substrate more than itself. We actually need the fluid to really like the substrate, or we risk adhesion failure after coating.
In more technical terms, the fluid and substrate both have an inherent amount of energy exhibited at the surface where they come in contact. If the energy within the fluid is high, relative to the substrate, the fluid will attract itself more and form droplets (i.e.: coating defects). If the energy of the substrate is high, relative to the fluid, the fluid will be unable to resist the attractive force of the substrate and be willing to wet-out the surface (i.e.: a good, coated product). The key is to encourage the fluid to like this new dissimilar material it is coming in contact with and spread evenly over a large surface area.
The rheology of the coating fluid can result in either dewetting and retraction with immiscible liquids (see Figure 2) or wetting and spreading with miscible liquids across a diffusion zone. Surface chemistry (contact angles and spreading coefficients) leads to understanding of the forces of physics that explain flow of the fluid after placement on a substrate.
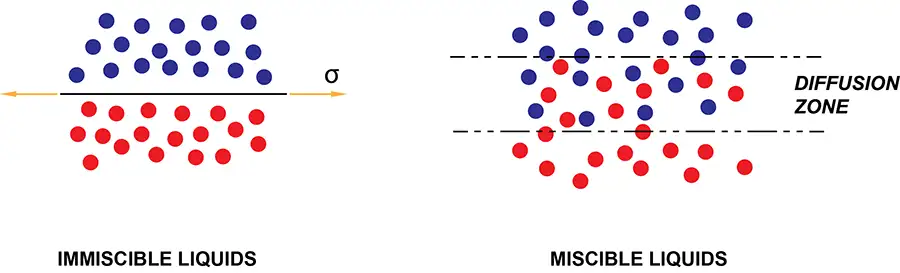
So, what’s going on?
If you are unlucky enough to have mismatched materials, what can you do? Let’s investigate what is going on. In a coating operation, the fluid meets the substrate at the location of the coating head. If the fluid and substrate-surface energies are near equilibrium, defects will occur at the interface. If we can increase the energy of the substrate, reduce the energy of the fluid or otherwise promote the marriage of the dissimilar materials, we win. To develop this harmonious matrimony of fluid and substrate, we can:
- Increase the surface energy of the substrate – Prior to the coating head, energy can be added to the substrate surface via corona, flame or plasma treatment. These treatments activate the end groups that attract the fluid, also called oxidizing.
- Decrease the surface energy of the fluid – Primer coating can act as a chemical binder for the fluid of interest and the substrate, creating a bridge for secure coating, or a surfactant can reduce the surface energy of the fluid, allowing it to wet-out over the substrate.
- Promote adhesion at the coating interface – Sometimes, the surface energy is only part of the reason for poor adhesion, and a vacuum at the coating head is enough to reduce the air barrier and allow for proper adhesion of the fluid to the substrate.
To know if you may have a problem before you have a problem, it is good to obtain the surface-energy information for both the fluid and the substrate. The manufacturer typically provides this in the product literature, or a test facility can determine the fluid’s surface tension. For the substrate, dyne pens can be purchased to provide an estimation of the surface energy, showing fluids of specific surface-energy levels, and you can observe the wettability at static conditions.
Getting stuff to stick
There are a variety of methods for encouraging fluids to stick to substrates in coating applications: 1) corona-, plasma- or flame-treating the substrate; 2) adding a primer prior to coating; and 3) using a vacuum system at the coating-head interface. The addition of surfactants is one method of adjusting the fluid itself that should be considered.
Especially with the use of aqueous fluids, surfactants provide the ability of the solution chemistry to adsorb at the substrate interface. This encouragement does not require a large dilution of the fluid with the surfactant additive, but it definitely provides a strong anchor for the water-based fluid to the substrate if nothing else is working.
Surfactants even out the flow of the fluid as it travels across the substrate. Especially when there is a quick expansion or contraction (like what is experienced in pre-metered coating methods), the fluid-surface deformation may vary across the substrate. This deformation results in concentration gradients and surface-tension gradients. These surface-tension gradients lead to variability in the coating. Surfactants help overcome these gradients and variations.
How surfactants work in surface treatment
But how does a surfactant provide this encouragement? The easiest way to think of a surfactant is as a collection of active monomers that bridge the fluid and the substrate on a moving web. Initially, the fluid is loaded with a predetermined amount of surfactant that has free-radical end groups. This means that the chemical additive (surfactant) has more energy to attach to the substrate surface than the polymer solution has on its own.
Because the surfactant is free to move about in the solution, the surfactant tends to migrate to the interface point where the fluid is in contact with the substrate, instead of staying in the bulk of the fluid. The diffusion of the surfactant monomer to the substrate, in essence, “grabs” the substrate surface, and the solution has an extended residence time on the substrate prior to drying and curing. This extra open time on the substrate allows the fluid to coat better and may reduce coating defects. This can be seen dramatically if you place a drop of fluid onto the substrate and it beads up without surfactant and then place a drop of the fluid with surfactant on the substrate and it wets-out and flows. The dramatic change of surface tension by a small volume of surfactant additive may be just what is required for improved coating performance.
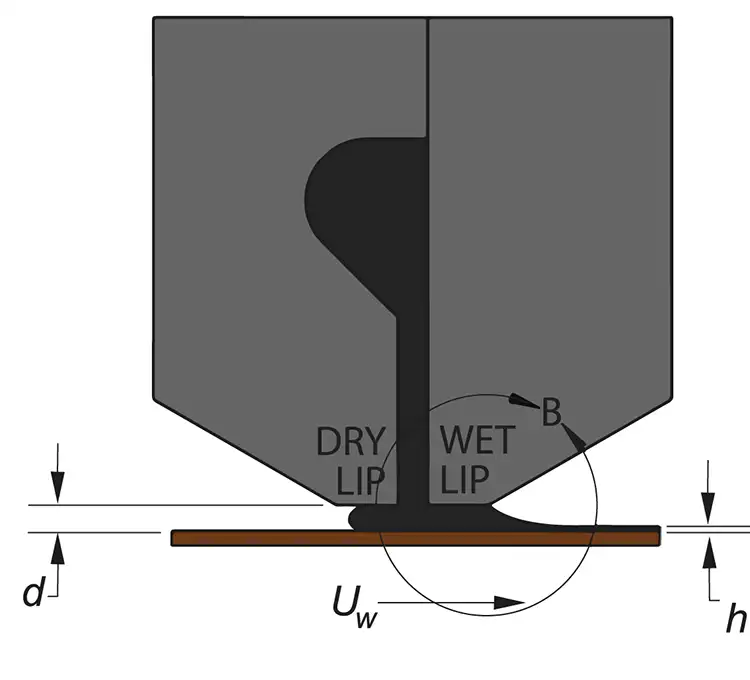
In pre-metered coating, the use of surfactants also can allow for a wider gap between the coating head and the substrate (see Figure 3). This can be considered a macro-sized bridge for the fluid and the substrate beyond the microscopic view we have already discussed. The advantages of this increased distance include coating a thinner fluid coating than normally would be allowed and reduction in the use of a vacuum system.
In addition to providing the connection between the liquid coating and the solid substrate that is required to make a viable coated product, surfactants sometimes can provide ancillary benefits, such as improved solubility for the solution or altering the surface roughness of the final coated product. Even though surfactants provide many benefits, their application should not be applied in a slapdash manner. Surfactants also have been recognized as creating coating defects, most noticeably hydrophobic spots. Proper use and application of surfactants can offer advantages to coating that cannot be accomplished otherwise.
Conclusion
The fluid-and-substrate marriage is tricky enough to encourage without the right energy present. Make these partners like each other more than they like themselves, and you have a coated product that will have fewer defects to overcome and a high likelihood of a persistent product. Happy matchmaking.
Mark Miller, CEO of Coating Tech Slot Dies LLC (Eau Claire, WI), holds a Bachelor’s in Chemical Engineering from the University of Wisconsin-Madison, a Master’s in Polymer Science and Technology from Lehigh University, and a J.D. from Hamline Mitchell School of Law. He has worked at 3M Co. and Coating Tech Slot Dies, and is a frequent presenter at AIMCAL R2R Conferences. Mark can be reached at 715-544-7568, mobile: 715-456-9545, mark.miller@slotdies.com or www.slotdies.com.

