By Richard P. Eckberg, Ph.D., chemical and converting industry consultant
Editor’s Note: This technical paper originally appeared in UV+EB Technology, 2021 Q3, pages 48-53. Part 1 covered the case for radiation-triggered, addition-cure, silicone-release agents.
Introduction
Silicone (polydimethylsiloxane) polymers are unique materials that are chemically inert and of low polarity that remain fluid at temperatures as low as -120° C and retain useful properties at temperatures >250° C for extended times. Cross-linked silicone polymers can be bulk-solid elastomers, rubbers, gels and low-surface-energy coatings capable of lightly adhering to pressure-sensitive adhesives and then releasing those adhesives without denigration of adhesive tack. Such coatings are known as release coatings. When applied to paper and film substrates as blends of reactive silicones, then cross-linked to a solid adhesive surface, the resultant silicone coating is the key element of a release liner.
Self-adhesive pressure-sensitive labels and laminated-label structures were first developed by R. Stanton Avery. The tag and label industry later adopted large-scale use of silicone-release liners as a way of packaging labels from time of manufacture to time of application. A familiar example of a release liner is the silicone-coated paper from which self-adhesive postage stamps are detached.
MeCpPtMe3 photocatalyzed silicone processing: Initial experiments and observations
Photo-hydrosilation experiments with MeCpPtMe3 were carried out using a model reaction to facilitate analysis [15].
Me3SiOSi(Me)[CH2CH2Si(Me)2OSi(Me)3]OSiMe3
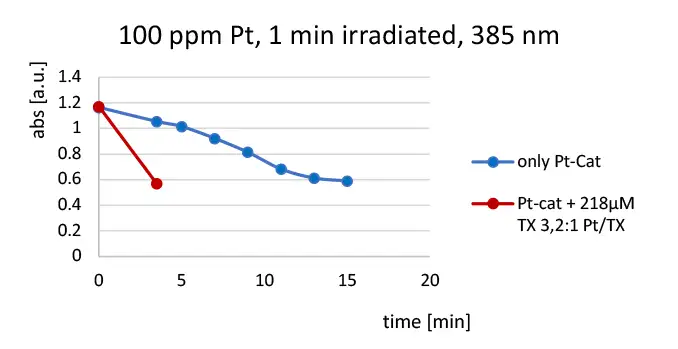
A 2:1 mix of 1,1,1,2,3,3,3 heptamethyltrisiloxane : vinylpentamethyldisiloxane was treated with sufficient MeCpPtMe3 to provide 100-ppm Pt, then irradiated with 385-nm LED radiation for 1 minute (see Figure 3). The reaction mixture was under N2. Loss of the catalyst 255-nm absorption peak was monitored as a function of irradiance time. The experiment was repeated with sufficient thioxanthone (TX) sensitizer added to provide ~30 mole% TX as a function of platinum.
No sensitization effect was observed when this experiment was repeated in the presence of oxygen. When the experiment was conducted tracking the 255-nm catalyst absorption peak after 10 seconds, irradiation from a standard medium-pressure Hg lamp, rapid initial loss of the absorption occurred followed by a long, slow continuing “dark” reaction. Sensitization was not apparent either inerted or not.
The model study results were consistent with earlier accounts of photo-hydrosilation catalyzed by MeCpPtMe3 but raised concerns that thioxanthones would not be effective sensitizing agents for photo-cross-linking of reactive silicone polymersin a non-inerted industrial coating operation. Of equal concern was the extent of “dark” reaction that would be considered a “post-cure,” meaning that cross-linking continues long after exposure to UV energy.
Hydrosilation is an exothermic process with a high heat of reaction of 28 kCal/mol [16] so photo-catalyzed hydrosilation is amenable to photo-DSC experimentation. Accordingly, representative coating formulations consisting of vinylsilicone and hydridosilicone polymers were treated with MeCpPtMe3 catalyst, then samples placed in a TA Instruments photo-calorimeter and irradiated with filtered UV energy in the 280- to 400-nm range that includes much of the catalyst absorption range.
Note that the initial exposure results in ~66% of total Delta H of available reaction. Five additional UV flashes were required to achieve > 90% cross-linking.
MeCpPtMe3 photocatalyzed silicone processing: Optimization experiments
Following the initial lab studies, we began trials of addition-cure, silicone release-coating formulas using a pilot UV processor at Heraeus Noblelight’s experimental facility in Gaithersburg, MD. The experiments consisted of making hand drawdowns of candidate catalyzed silicone coatings on sheets of Verso™ SCK paper-liner substrate, then passing the coated sheets on a conveyer under one or two focused microwave-fired, mercury-vapor lamps. Coatings were then evaluated for silicone transfer to, and detackification of, 3M #610 cellophane tape (evidence of incomplete cure), obvious finger smear, and rub-off from the substrate. Cure was defined on an arbitrary scale of 0 to 4, with 0 = no cure (wet) and 4 = well cured (no migration, no smear, good anchorage). Commercial release-coating cure of at least “3” is a promising result. Coating variables included catalyst concentration, SiH/vinyl molar coating ratio, anchorage additive, sensitizer content; processing variables included lamp choice, lamp reflector type and conveyer speed. Achieving a cure score of 3 or 4 under one curing lamp at 200 fpm conveyer speed was a goal of these experiments.
There is a significant “red shift” in spectral output of an iron-doped, mercury D lamp versus the conventional medium-pressure mercury-vapor bulb, so we felt it worthwhile to compare cure response as a function of lamp type, because the absorption spectrum of MeCpPtMe3 trails well into the UVA region. Our results are summarized in Figure 4.
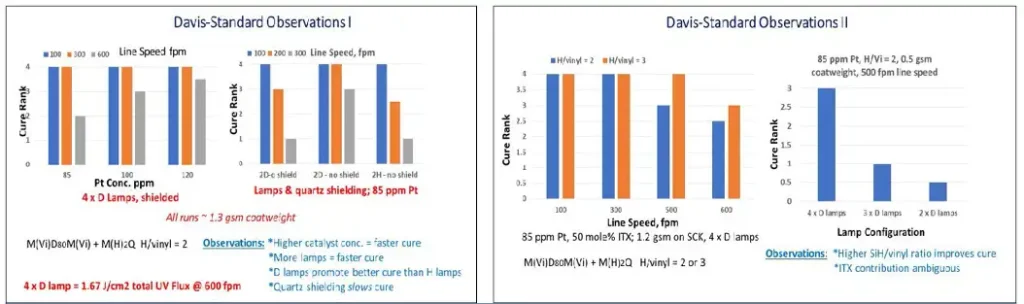
We were surprised by the significant improvement in cure speed provided by use of a D lamp in place of the H lamp. Additional improvement is provided by use of dichroic reflectors used to filter out IR frequencies. When well cured, all coatings tested OK as release coatings, but quantitative degree of curecould not be assessed.
The next set of experiments was run as coating trials on the five-roll pilot, differential coater at the tandem coating lab of Davis-Standard LLC, in Fulton, NY. Candidate silicone-coating formulas were applied at ~1 to 1.3 gsm coatweight (~1 to 1.3 microns thick) onto Verso SCK paper-liner sheet. Vinyl silicone polymers and hydrido silicone cross-linking polymers were mixed with MeCpPtMe3, anchorage additive and isopropylthioxanthone (ITX) sensitizer.
The pilot-coating lines at Davis-Standard are equivalent to commercial converting operations in terms of silicone-coatweight control, line speed and ability to apply silicone-release coating to a wide range of substrates. The observed photocure of hydrosilation cross-linked silicones at near-commercial line speeds was promising, but the requirement of four sets of microwave-fired D lamps delivering a total of 1.7 J/cm2 UV flux (at 600-fpm line speed) to these coatings to overcome an inherent slow activation of MeCpPtMe3 catalyst is an expensive fix. Additional trials at Davis-Standard were subsequently carried out to determine how to further improve photo-response of the system and to acquire quantitative degree of cure data.
Extractable silicone is a precise determination of uncross-linked silicone extant in a release coating after processing (see Figure 5). It is measured by immersing a precisely sized sample of the coated substrate in a precisely measured amount of methyl-isobutyl ketone for >24 hours, then using AA spectroscopy to measure siliconcontent of the extractant solvent. The AA result translates to % of uncured silicone extracted. Industry requirement is typically 5% maximum from a liner within a few minutes of cure. Greater than 10% extractable silicone results in backside transfer of silicone on rewind and indicates likely loss of adhesive tack from PSAs laminated to the liner.
Dual cure: Key to MeCpPtMe3 photocatalyzed silicone-release coatings?
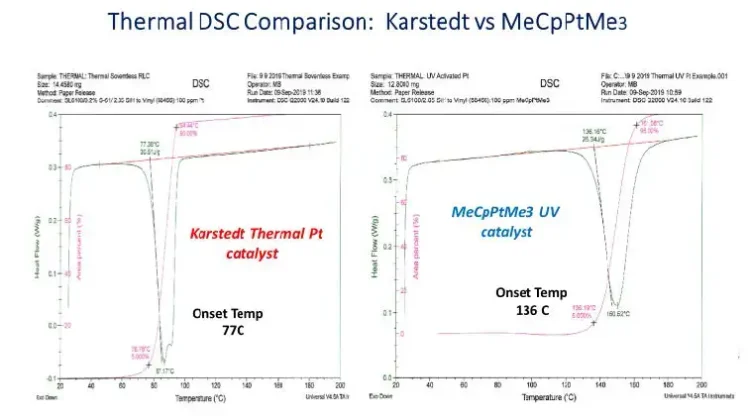
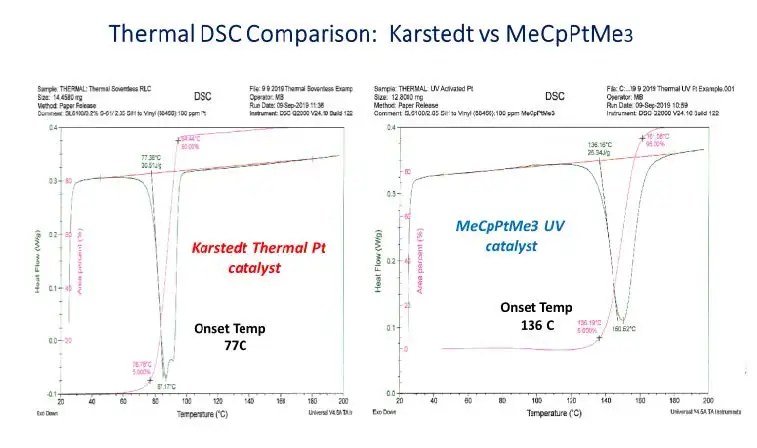
An assumption underlying the project to develop photo-activated, addition-cure, silicone-release coatings is that MeCpPtMe3 photocatalyst is strictlya photocatalyst that is stable at ambient conditions in a reactive silicone-coating formulation including vinyl- and hydrido-silicone. But thermalDSC traces of identical polymer blends catalyzed by inhibited Karstedt and inhibitor-free MeCpPtMe3 catalysts demonstrate that the photo-active compound also acts as a thermal catalyst for hydrosilation (see Figure 6).
These DSC results show that, while MeCpPtMe3 is a very stable catalyst kept in the dark at 25° C, it will function as a thermallyactivated hydrosilation catalyst at sufficiently elevated temperature. This observation is consistent with studies of the mechanism of CpPtR3 catalysis of hydrosilation showing that absorption of light leads to breaking a strong sigma bond between Pt and alkyl ligands with loss of alkane to open the Pt coordination sphere [17], a slow rate-determining step that may account for long post-cure versus Karstedt complex. We next ran simple isothermal photo-DSC studies (as described previously) of the most favorable coating blend per the Davis-Standard trials at different temperatures to see how heating the coating affected photo-response. Two photo-DSC traces of the same 50-ppm Pt formula are depicted in Figure 7. Note that initial 0.6-second UV exposure results in <50% cross-linking at 25° C but provides ~85% cross-linking when the coating is heated to 70° C. Repeating this Photo-DSC experiment at intermediate isothermal conditions between 25° C and 70° C confirmed that coating temperature is related directly to speed of cross-linking.

Conclusions and prospects
Photo-activated hydrosilation of reactive vinylsilicone + hydridosilicone polymers is catalyzed by cyclopentadienyl-Pt(IV) alkyl complexes, most notably MeCpPtMe3, but a UV-curable, silicone-coating system based on this catalyst requires very high UV flux and optimal substrate selection and may be suitable only for certain specialty silicone release-coating applications.
Our studies have shown that favorable combinations of vinylsilicone and hydride-functional silicone polymers, coupled with certain types of curing lamps, provide the best performance at realistic catalyst concentration. The next step in this project will be to combine a mild heat stage with UV irradiation. Temperatures in the 75° C to 100° C range will not degrade film or paper substrates if such conditions enable rapid, complete photo-cross-linking on exposure to two banks of lamps on high-speed coating lines – a dual-cure means of manufacturing new types of thinner, dimensionally stable, premium release liners will be realized.
References
15. T. Holzel, Master’s Thesis Universitat Dusseldorf 2016
16. Silicone Surfactants, Randal Hill, Ed., Marcel Dekker, New York 1999
17. Jakubek and Lees, Inorg. Chem. Comm. 2004 (43), 6869

Richard Eckberg, Ph.D., a chemical and converting industry consultant based in Hillsborough, NC, holds an A.B. degree in Chemistry from Hamilton College and a Ph.D. in Inorganic Chemistry from the University of North Carolina-Chapel Hill. He held Product Development and Technical Marketing positions with General Electric Silicones and Momentive Performance Materials from 1976 to 2020, focusing on silicone-release agents and energy-curable (UV & EB) silicones and related materials. During his +40-year career, Rick has authored and co-authored over 70 US and foreign patents, published numerous articles in trade, technical, and scientific journals, contributed chapters to various textbooks and has been a frequent speaker at international industry forums sponsored by RadTech International, the American Chemical Society, and Adhesion Society among others. He is a US Army veteran and professional musician. Rick can be reached at 919-929-0861, mobile: 919-357-1870 or email: rickeckberg@nc.rr.com.

