By Richard P. Eckberg, Ph.D., chemical and converting industry consultant
Editor’s Note: This technical paper originally appeared in UV+EB Technology, 2021 Q3, pages 48-53.
Introduction
Silicone (polydimethylsiloxane) polymers are unique materials that are chemically inert and of low polarity that remain fluid at temperatures as low as -120° C and retain useful properties at temperatures >250° C for extended times. Cross-linked silicone polymers can be bulk-solid elastomers, rubbers, gels and low-surface-energy coatings capable of lightly adhering to pressure-sensitive adhesives and then releasing those adhesives without denigration of adhesive tack. Such coatings are known as release coatings. When applied to paper and film substrates as blends of reactive silicones, then cross-linked to a solid abhesive surface, the resultant silicone coating is the key element of a release liner.
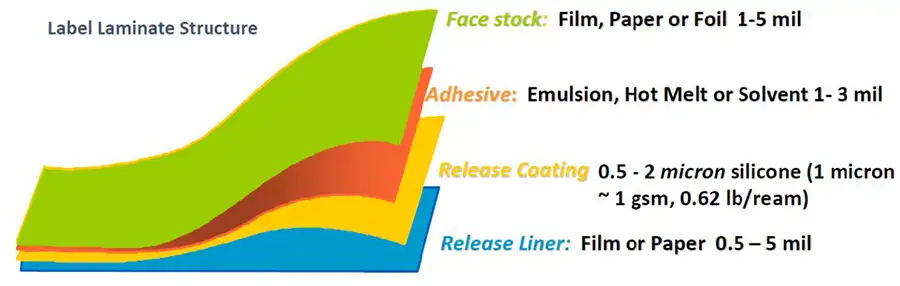
Self-adhesive pressure-sensitive labels and laminated-label structures were first developed by R. Stanton Avery [1,2]. The tag and label industry later adopted large-scale use of silicone-release liners as a way of packaging labels from time of manufacture to time of application. A familiar example of a release liner is the silicone-coated paper from which self-adhesive postage stamps are detached. Figure 1 depicts the structure of conventional label laminate. Note that the silicone-release coating is very thin relative to the rest of the construction.
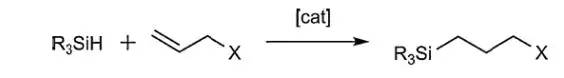
Catalytic addition of silicon hydrides to olefins and acetylenes is the basis of manufacture of organofunctional silanes and silicones. This hydrosilation reaction in its simplest form can be depicted as Notation 1.
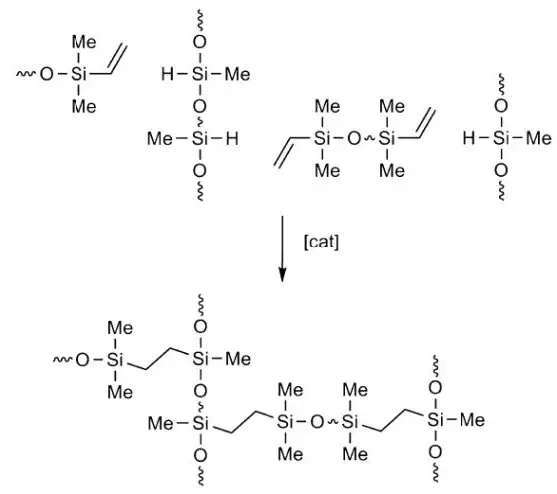
Numerous transition metal compounds and complexes are known to catalyze this reaction [3], but industrial processes mostly use silicone-soluble platinum (II) complexes in homogeneous phase because of the speed and anti-Markovnikov stereospecificity afforded by these catalysts. Most silicone-release coatings are applied to paper and film webs or sheets as 100- to 500-cstk viscosity reactive fluid blends consisting of vinyl-functional, silicone-base polymers and hydride-functional, silicone-crosslinker polymers with hydrosilation catalysts, inhibitors and other additives present (see Notation 2).
Karstedt [4] type Pt catalyst is a very reactive silicone-miscible Pt(II) complex in common use for thermal processing of silicone-release coating agents (see Notation 3). It is necessary to include an inhibitor that slows the curing reaction enough at ambient temperatures to permit coating [5]; useful inhibitors are volatile and are blown out of the silicone coating in high-temperature ovens immediately after application, thus permitting cure. Processing of silicone-release coatings at very high speeds (>1,000 mpm) is practiced, but doing so requires sufficient heat to raise substrate and coating temperatures >150° C, which demoisturizes paper liners and deforms and degrades film and film-laminate liners, leading to liner dimensional instability after processing.
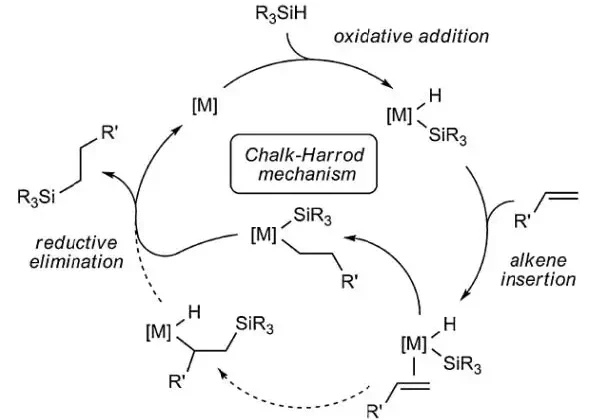
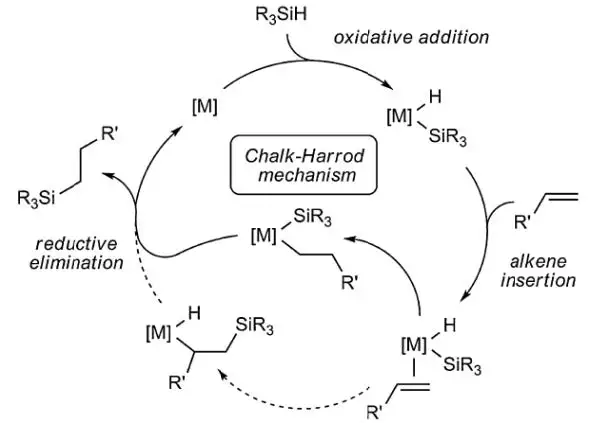
The mechanism of Karstedt-catalyzed hydrosilation has been well elucidated; the Chalk-Harrod [6] mechanism is widely understood to be a good explanation of how silicone addition-cure operates at the molecular level (see Notation 4).
The case for radiation-triggered addition-cure silicone-release agents
High-speed reliable processing of silicone-release liners that takes advantage of Pt(II)-catalyzed, thermal addition-cure chemistry is well established but limits choice of liner substrates to robust paper sheets >40 lb/ream basis weight that require sufficient heat to reach web temperatures that promote complete cross-linking of reactive silicone polymers. Thermal addition-cure chemistry largely rules out use of dimensionally stable, thin-gauge polyolefin and PET liners because such substrates degrade and deform at temperatures as low as 110° C.
To meet demand for low-temperature curing, silicone-release coatings, free-radical and cationic-type UV-curable acrylate- and cycloaliphatic epoxy-functional, silicone polymers and compatible photocatalysts have been developed [7,8], and have found commercial specialty market niches. Such coatings require organofunctional silicones that use expensive inputs, complex multistep syntheses and silicone-compatible photocatalysts – all of which make these coatings at least twice as expensive as their simple addition-cure silicone analogs. In addition, free-radical photocure of acrylated silicones runs at commercial speeds only in a rigidly inerted atmosphere (<50 ppm O2), adding more cost and complexity to processing. To build sufficient reactivity to provide fast photocure into these types of silicone coatings, >15% (w/w) of the polymers consists of polar, reactive non-silicone organofunctionality, which compromises release of aggressive mastic- and acrylic-based, pressure-sensitive adhesives. An ideal low-temperature, radiation-curable silicone coating would be a 100% addition-curable silicone whose cure is triggered by exposure to ultraviolet or electron-beam (UV or EB) energy.
UV-active hydrosilation catalysts
There are numerous accounts of research extant in the literature describing various transition metal compounds and complexes capable of catalyzing hydrosilation reactions upon absorption of incident UV energy [3], but the only compounds described that appear fast and efficient enough for possible use in high-speed coating operations for release coatings are certain photo-active Pt(II) and Pt(IV) complexes. Most promising are cyclopentadienyl Pt(IV) compounds.
In the 1980s, researchers at 3M patented the use of certain eta-cyclopentadienyl (Cp) platinum (IV) compounds as photocatalysts for hydrosilation [9,10] and later expanded the concept to include such Pt(IV) compounds in conjunction with sensitizers to expand the range of input light that could trigger hydrosilation curing reactions [11]. A subsequent patent describes modified Cp-Pt(IV) compounds that incorporate near UV/VIS light-absorbing functional groups [12] with the goal of making an addition-cure process amenable to long-wavelength UV or visible light activation. Concerns about the volatility of methyl-cyclopentadienyl Pt(IV) trimethyl (MeCpPtMe3) led to attachment of hydrolysable siloxy groups on the Cp ligand [13].
There are three main features of the UV-absorption spectrum of MeCpPtMe3: A major UV-C peak centered ~255 nm, a secondary shoulder near 289 nm and a long tail extending into UVA region above 350 nm, suggesting that conventional medium-pressure, mercury-vapor, UV-curing lamps ought to be suitable for photocure of vinylsilicone + hydride-silicone coatings. Given the widespread availability of such lamps and UV-cure coating lines equipped with them, as well as the considerable patent literature teaching use of MeCpPtMe3 for photo-hydrosilation, it is surprising that UV-activated, addition-cure release coatings are not established commercial products. MeCpPtMe3 is used for photo-catalysis of silicone gels and elastomeric conformal coatings [14], but these are thick-section, slow-curing articles with very low catalyst content requiring long UV-exposure times not feasible for a high-speed converting operation.
References
- S. Avery, US Patent 2,304,787 (12/15/1942)
- S. Avery, US Patent 2,783,172 (2/26/1957)
- R. Hofmann et al, Polymers 2017 (9), 534
- B. Karstedt, US Patent 3,715,334 (2/6/1973)
- R. Eckberg, US Patent 4,256,870 (3/17/1981)
- A. Chalk and J. Harrod, J. Amer. Chem. Soc. 1965 (87), 16
- O. Pinto et al, US Patent 6,548,568 (4/15/2003)
- R. Eckberg et al, US Patent 4,279,717 (7/21/1981)
- T. Drahnak, US Patent 4,510,094 (4/9/1985)
- T. Drahnak, US Patent 4,600,484 (7/15/1986)
- L. Boardman et al, US Patent 4,916,169 (4/10/1990)
- M. Butts, US Patent 6,451,869 (9/17/2002)
- A. Koellnberger US Patent 8,088,878 (1/3/2012)
- Silopren™ UV-Cure Silicone Elastomers (Momentive Performance Materials)
Part 2 of this technical paper will cover MeCpPtMe3 photocatalyzed silicone processing experiments and observations, as well as optimization and conclusions.

Richard Eckberg, Ph.D., a chemical and converting industry consultant based in Hillsborough, NC, holds an A.B. degree in Chemistry from Hamilton College and a Ph.D. in Inorganic Chemistry from the University of North Carolina-Chapel Hill. He held Product Development and Technical Marketing positions with General Electric Silicones and Momentive Performance Materials from 1976 to 2020, focusing on silicone-release agents and energy-curable (UV & EB) silicones and related materials. During his +40-year career, Rick has authored and co-authored over 70 US and foreign patents, published numerous articles in trade, technical and scientific journals, contributed chapters to various textbooks and has been a frequent speaker at international industry forums sponsored by RadTech International, the American Chemical Society and Adhesion Society among others. He is a US Army veteran and professional musician. Rick can be reached at 919-929-0861, mobile: 919-357-1870, email: rickeckberg@nc.rr.com.

